Joseph Levine, M.D., Yoram Barak, M.D., Mirtha Gonzalves, M.D., Henry Szor, M.D., Avner Elizur, M.D., Ora Kofman, Ph.D., and R.H. Belmaker, M.D.
Received Aug. 26, 1993; revision received Feb. 3, 1994; accepted April 22, 1994. From the Yehuda Abarbanel Mental Health Center, Bat Yam, Israel; and the Ministry of Health Mental Health Center, Faculty of Health Sciences and Depamnent of Behavioral Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel. Address reprint requests to Dr. Belmaker, Beer-Sheva Mental Health Center, P.O. Box 4600, Beer-Sheva, Israel.
Supported by contract 263-MD-340-3442 from the NIMH New Medications Development Office.
OBJECTIVE: CSF levels of inositol have been reported to be lower than normal in depressed subjects. The authors administered inositol to depressed patients in a double-blind, controlled trial. METHOD: Under double-blind conditions, 12 g/day of inositol (N=13) or placebo (N=25) was administered to depressed patients for 4 weeks. RESULTS: The overall improvement in scores on the Hamilton Depression Rating Scale was significantly greater for inositol than for placebo at week 4. No changes were noted in hematology or in kidney or liver function. CONCLUSIONS: This may be the first use of the precursor strategy for a second messenger rather than a neurotransmitter in treating depression. Although inositol had a significant antidepressant effect in this study, replication is crucial. (Am J Psychiatry 1995; 152:792-794)
Inositol is a simple isomer of glucose that is a key metabolic precursor in the phosphatidylinositol cycle. Unlike L-dopa and tryptophan, which are amino acid precursors of monoamine neurotransmitters and which have been reported to have antidepressant properties, inositol is a precursor of an intracellular second messenger system. The phosphatidylinositol cycle is a second messenger system for numerous neurotransmitters (1).
Barkai et al. (2) reported that depressed patients, both unipolar and bipolar, had markedly low levels of inositol in CSF. In an open study of 11 depressed patients who had been resistant to previous antidepressant treatment (3), inositol treatment led to a decline in mean Hamilton Depression Rating Scale score from 31.7 (SD=6) to 16.2 (SD=9). Levine et al. (4) showed that 12 g/day of inositol raised CSF inositol levels by 70%. We report here the results of administering 12 g/day of inositol to depressed patients in a double-blind, controlled trial.
METHOD
The diagnostic entry criteria for the study were DSM-III-R major depression or bipolar disorder, depressed. Each of the patients who were referred to the study and gave informed consent had not re- sponded to antidepressant treatment, had dropped out of treatment because of side effects, or, in the case of the bipolar depressed patients, were lithium prophylaxis patients who had had few problems with mania but intolerable continuation of depression. All medications other than study drugs were stopped at least 3 days, and usually 1 week, before the subjects entered the study. No medications other than inositol or placebo were permitted during the trial, except for oxazepam, up to 15 mg/day, or an equivalent benzodiazepine if the patient had been taking it before the study. The study was approved by the Helsinki Committee institutional review board and the Ministry of Health. Each patient gave written informed consent before participation.
Thirty-nine patients entered the trial. Of these, 11 dropped out within 1 week of starring the trial. Four of these patients were taking placebo, and their reasons for dropping out, respectively, were headache, hypomania, insomnia, and lack of desire to continue in the study. Seven patients taking inositol dropped out within 1 week; two had mild psychotic symptoms, one had weakness and tremor, one had a cutaneous burning sensation, and three did not want to continue in the study. These 11 patients are not included in the data analysis. One patient taking inositol dropped out after 3 weeks because of total remission of symptoms, and her 2-week score was used as a last value carried forward.
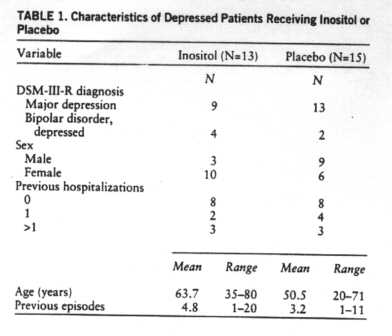
The mean length of the current depressive episode for the 13 patients taking inositol who completed the study was 3.9 months (SD=3), and for the 15 patients taking placebo it was 5.4 months (SD-3). Other characteristics are shown in table 1.

All patients were given inositol or glucose in identical containers according to a prearranged random code. The drug was in powder form, and the patients were instructed to take 2 teaspoons morning and evening in juice. A modified Treatment Emergent Symptom Scale was used to monitor side effects. Hematology, blood chemistry, liver function, and kidney function were assessed at baseline and after 4 weeks of inositol treatment.
RESULTS
In the placebo group, the mean Hamiiton depression scale score declined from 32.9 (SD=5) at baseline to 29.2 (SD=7) at 2 weeks and 28.9 (SD=1O) at 4 weeks. In the inositol group, it declined from 33.4 (SD=6) at baseline to 27.3 (SD=8) at 2 weeks and 21.6 (SD=10) at 4 weeks. Individual values are shown in figure 1.
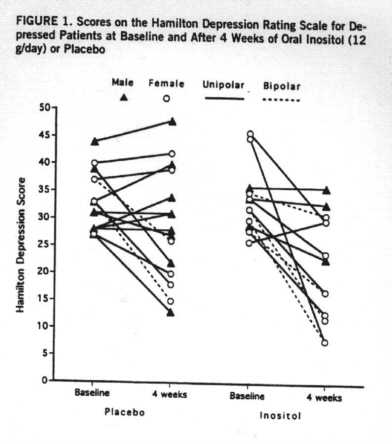
Analysis of variance of the final improvement scores (baseline minus week 4) for all subjects showed that inositol reduced the Hamilton depression score significantly more than did placebo (F=4.48, df=1, 26, p=0.04). After 2 weeks of treatment there was no difference in the improvement scores of the placebo and inositol groups.
Male patients showed little improvement, whether treated with inositol (mean improvement=2.3, SD=3.2; N=3) or placebo (mean improvement=1.7, SD=8.6; N= 9). The mean 4-week improvement score of the two female bipolar patients receiving placebo (mean=14.5, SD=4.9) was nearly equal to that of the four female bipolar patients receiving inositol (mean=14.3, SD=8.3). When improvement was defined as a decrease of 15 or more points on the Hamilton depression scale, three of the 15 patients taking placebo and six of the 13 patients taking inositol were shown to have improved. Six patients taking placebo and one taking inositol showed worsening during the trial, i.e., an increase in Hamilton scale score.
Side effects experienced by patients in the placebo group were agitation and tension (N=1), headache (N=1), and insomnia (N=1). In the inositol group there were complaints of nausea (N=1) and flatus (N=1). There were no abnormalities in urea, sodium, potassium, calcium, phosphate, creatinine, alkaline phosphatase, SGPT, SGOT, creatine phosphokinase, LDH, urinalysis, CBC, or differential count. Two patients showed a mild increase in fasting blood sugar after 4 weeks of inositol; the increase disappeared in one patient upon repeat testing, although the patient continued (at her request) to receive open inositol treatment. The other patient showed the same mild increase several weeks after discontinuation of inositol.
DISCUSSION
The present study is, to our knowledge, the first use of the precursor strategy for a second messenger rather than a neurotransmitter (5) in treating depression. As with other precursors, such as L-dopa for dopamine or tryprophan for serotonin, doses of several grams per day seem necessary for CNS effects of inositol (4). Since we found no major clinical side effects of inositol and no laboratory changes in kidney, liver, or hematologic measures, in the future investigators should consider inositol doses of 20-30 g/day. Since we used 3 g per teaspoon dissolved in juice and since 4 teaspoons dissolve easily in a glass of juice, these doses could conceivably be well tolerated. Arendrup et al. (6) gave 20 g/day of inositol to diabetics without side effects. Hallman et al. (7) gave 160 mg/kg of inositol per day to neonates. However, caution is indicated since some studies suggest that severe hyperinositolemia may cause reductions in peripheral nerve conduction (8).
An important observation in the present study was the absence of manic episodes in the bipolar depressed patients treated with inositol. Considerable evidence suggests that lithium acts by reducing inositol levels at some intracellular brain site (5). This would reduce the functional activity of a hypothetical overactive receptor in mania. The present results suggest that when these hypothesized signaling systems are not overactive, additional inositol does not increase their activity.
The present finding that inositol is more effective than placebo in reducing scores on the Hamilton Depression Rating Scale in depressed patients depends on the subgroup of female unipolar patients. This subgroup effect may point to a real biological distinction, or it may represent a chance association between the unipolar/bipolar distinction or male/female distinction and inositol response. Levine et al. (3), in an open study of 11 unipolar depressed patients, found excellent responses to inositol in male and female patients. While the potential antidepressant effect of inositol is a novel finding, the number of subjects in the present study was small and replication is crucial.
REFERENCES
1. Baraban JM, Worley PF, Snyder SH: Second messenger systems and psychoactive drug action: focus on the phosphoinositide system and lithium. Am J Psychiatry 1989; 146:1251-1260
2. Barkai IA, Dunner DL, Gross HA, Mayo P, Fieve RR: Reduced myo-inositol levels in cerebrospinal fluid from patients with affeccive disorder. Biol Psychiatry 1978; 13:65-72
3. Levine J, Gonzalves M, Babur I, Stir S, Elizur A, Kofman O, Belmaker RH: Inositol 6 gm daily may be effective in depression but not in schizophrenia. Human Psychopharmacoiogy 1993; 8: 49-53
4. Levine J, Rapaport A, Lev L, Bersudsky Y, Kofman O, Belmaker RH, Shapiro J, Agam G: Inositol treatment raises CSF inositai levels. Brain Res 1993; 627:168-170
5. Kofman O, Belmaker RH: Biochemical, behavioral and clinical studies of the role of inositol in lithium treatment and depression. Biol Psychiatry 1993; 34:839-852
6. Arendrup K, Gregersen G, Hawley J, Hawrhorne JN: High-dose dietary myo-inositol supplementation does not alter the ischemia phenomenon in human diabetics. Acta Neurol Scand 1989; 80:99- 102
7. Hallman M, Jarvenpaa AL, Pohjavuori, M: Respiratory distress syndrome and inositol supplementation in preterm infants. Arch Dis Child 1986; 61:1076-1083
8. Clements RS Jr, De Jesus PV, Winegrad AI: Raised plasma myoinositol levels in uraemia and experimental neuropathy. Lancet 1973; 1:1137-1141