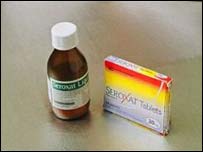
Seroxat is used in the treatment of depression
and anxiety disorders |
Young
people under the age of 18 should not be prescribed the
controversial drug Seroxat, government advisors have ruled.
It follows a review which found children taking the
anti-depressant may be more likely to self-harm or partake in
suicidal behaviour.
The Medicines and Healthcare products Regulatory Agency (MHRA)
has also warned that adults who are on the drug should not suddenly
stop taking it.
Authorities in Europe and the United States are expected to
review their advice on Seroxat in light of the agency's findings.
Side-effect claims
The Department of Health launched the review of Seroxat and
similar drugs, known as SSRIs (Selective Serotonin Re-uptake
Inhibitors) in January.
It followed claims from patient groups that the drug has serious
side-effects and is addictive.
A recent investigation by the BBC's Panorama programme also
raised concerns about the drug.
 Young people under 18 years currently taking
Seroxat for depression should consult their doctor Young people under 18 years currently taking
Seroxat for depression should consult their doctor 
|
Seroxat has been available in the UK for the past 13 years.
Approximately four million prescriptions for the drug were issued in
the past year.
An estimated 8,000 patients under the age of 18 have been treated
with the drug over the last 12 months.
This is despite the fact that the drug is not licensed for use in
under 18s. However, doctors can prescribe Seroxat to people in this
age group if they believe it is in the patient's best interest.
But the review, by the Committee on Safety of Medicines (CSM),
has concluded the drug should no longer be prescribed to children.
Its experts said the risks outweighed the potential benefits.
Their decision was based on new research provided by
GlaxoSmithKline, the makers of Seroxat.
It showed that the drug was not effective at treating depression
in under 18s.
Its studies on more than 1,000 children also suggested those on
Seroxat were at least twice as likely to have suicidal thoughts or
self-harm compared to children with similar mental health problems
who are not taking the drug.
Glaxo submitted the information to the agency in May.
Professor Gordon Duff, chairman of the CSM, said Seroxat should
no longer be prescribed to under 18s.
"Seroxat is not licensed for use in children but we know it is
used in this age group outside its licensed indications where
prescribers make a judgement on their own responsibility that it is
the right treatment for a particular patient.
"It is therefore important that doctors, patients and parents are
aware of the new advice.
"Young people under 18 years currently taking Seroxat for
depression should consult their doctor."
Further review
Professor Ian Weller, who headed the review into the drug, said
they would now examine whether it should continue to be prescribed
to adults.
"The expert group will be examining urgently what implications,
if any, these new findings have for the use of Seroxat in adults.
 This treatment has proven effective and has
helped millions of people around the world This treatment has proven effective and has
helped millions of people around the world 
Dr Alastair Benbow,
GlaxoSmithKline
|
"CSM has advised that at present the evidence is not sufficient
to confirm a causal association between SSRIs and suicidal behaviour
in adults.
"The benefits of taking Seroxat are well established and patients
over 18 years and those who are benefiting from Seroxat should not
be frightened into stopping their medication."
GlaxoSmithKline welcomed the decision and said adults should
continue taking the drug.
Dr Alastair Benbow, its head of European Psychiatry, said:
"Today's decision by the MHRA is solely concerned with the treatment
of children and teenagers under 18 years with major depressive
disorder.
"It is not related to the use of Seroxat by adults, where this
treatment has proven effective and has helped millions of people
around the world to lead fuller and more productive lives."
Richard Brook, chairman of the mental health charity, MIND
welcomed the ruling.
"MIND has been concerned to ensure that the experiences of users
of Seroxat were taken seriously.
"This new evidence underlines the concerns that young patients
have been voicing and MIND accept that speedy action has been taken
to address this new evidence.
"MIND strongly believes that the decision today requires us to
move very quickly forward on the review of Seroxat and other SSRIs
especially given the strength of concern we've heard from people
taking these drugs."
The Royal College of Psychiatrists welcomed the new advice from
the MHRA and said it would ensure it was passed on to its members
promptly.
It said it had been concerned for many years about prescribing of
anti-depressants for under 18s and had raised the matter with the
Department of Health.
