|
| ||
Vol. 286, Issue 3, 1474-1481, September 1998
Department of Pharmacology and Experimental Therapeutics, Loyola University of Chicago, Stritch School of Medicine, Maywood, Illinois
| |
Abstract |
|---|
The present study provides the first autoradiographic evidence of
age-dependent regional changes in the density of serotonin (5-HT)
transporters in offspring following prenatal exposure to fluoxetine.
Pregnant rats received either saline or fluoxetine (10 mg/kg,
s.c.) daily from gestational day 13 through 20. The density
of [3H]citalopram-labeled 5-HT transporters was determined in
forebrain regions and in midbrain raphe nuclei of prepubescent and
adult male offspring. Brain regions representing integral
components of the limbic system were particularly sensitive to the
prenatal treatment. For example, prenatal fluoxetine exposure
significantly altered the density of 5-HT transporters in subregions
of the hypothalamus (dorsomedial nucleus, 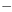 21%; lateral hypothalamus, +21%), hippocampus (CA2,
+47%; CA3, +38%), and amygdala (basolateral nucleus, +32%; medial
nucleus, +44%) in prepubescent offspring. However, 5-HT transporter
density in the dorsal and median raphe was unaltered in this same
group of offspring. In adult offspring, 5-HT transporter densities,
in all brain regions examined, were not significantly altered by
prenatal exposure to fluoxetine. The present study also identifies
significant age-related differences in 5-HT transporter densities
between prepubescent and adult control offspring. For example, in
adult control offspring, densities of 5-HT transporters were
significantly greater in the cingulate cortex (+33%), basolateral
amygdala (+58%), and CA1 area of the hippocampus (+78%); but
significantly lower in the temporal cortex (
21%; lateral hypothalamus, +21%), hippocampus (CA2,
+47%; CA3, +38%), and amygdala (basolateral nucleus, +32%; medial
nucleus, +44%) in prepubescent offspring. However, 5-HT transporter
density in the dorsal and median raphe was unaltered in this same
group of offspring. In adult offspring, 5-HT transporter densities,
in all brain regions examined, were not significantly altered by
prenatal exposure to fluoxetine. The present study also identifies
significant age-related differences in 5-HT transporter densities
between prepubescent and adult control offspring. For example, in
adult control offspring, densities of 5-HT transporters were
significantly greater in the cingulate cortex (+33%), basolateral
amygdala (+58%), and CA1 area of the hippocampus (+78%); but
significantly lower in the temporal cortex (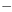 65%) and median raphe (
65%) and median raphe (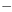 25%). The age-dependent and site-specific alterations
in the density of 5-HT transporters suggests that either 5-HT
innervation and/or 5-HT neuron function in various forebrain regions
may be altered by prenatal exposure to fluoxetine.
25%). The age-dependent and site-specific alterations
in the density of 5-HT transporters suggests that either 5-HT
innervation and/or 5-HT neuron function in various forebrain regions
may be altered by prenatal exposure to fluoxetine.
| |
Introduction |
|---|
Fluoxetine selectively inhibits the reuptake of serotonin (5-HT) into
presynaptic nerve terminals thereby increasing synaptic
concentrations of 5-HT (Fuller et al., 1991 ; Thomas et al., 1987
; Thomas et al., 1987 ). Repeated administration of fluoxetine to adult male
rats has been demonstrated to markedly alter serotonergic
neurotransmission as a result of fluoxetine-induced changes in brain
5-HT metabolism and 5-HT receptor density and function (Caccia et
al., 1992
). Repeated administration of fluoxetine to adult male
rats has been demonstrated to markedly alter serotonergic
neurotransmission as a result of fluoxetine-induced changes in brain
5-HT metabolism and 5-HT receptor density and function (Caccia et
al., 1992 ; De Montigny et al., 1990
; De Montigny et al., 1990 ; Li et al., 1993
; Li et al., 1993 ; Welner et al., 1989
; Welner et al., 1989 ; Wong and Bymaster, 1981
; Wong and Bymaster, 1981 ). Indeed, the therapeutic efficacy of fluoxetine is
believed to be mediated by the drug's ability to enhance serotonergic
neurotransmission upon repeated administration (Goodwin, 1996
). Indeed, the therapeutic efficacy of fluoxetine is
believed to be mediated by the drug's ability to enhance serotonergic
neurotransmission upon repeated administration (Goodwin, 1996 ; Owens, 1996, 1997
; Owens, 1996, 1997 ). Due to its relative selectivity, efficacy and safety
in humans, (Fuller et al., 1991
). Due to its relative selectivity, efficacy and safety
in humans, (Fuller et al., 1991 ; Thomas et al., 1987
; Thomas et al., 1987 ; Wong et al., 1991
; Wong et al., 1991 ) fluoxetine is routinely prescribed to millions of
Americans each year, including women of reproductive age, for the
treatment of a variety of psychiatric disorders (Dubovsky, 1994
) fluoxetine is routinely prescribed to millions of
Americans each year, including women of reproductive age, for the
treatment of a variety of psychiatric disorders (Dubovsky, 1994 ; Hudson et al., 1996
; Hudson et al., 1996 ; Kessler et al., 1993
; Kessler et al., 1993 ; Sheehan and Harnett-Sheehan, 1996
; Sheehan and Harnett-Sheehan, 1996 ). Consequently, many women may continue to be treated with
fluoxetine during pregnancy (Chambers et al., 1996
). Consequently, many women may continue to be treated with
fluoxetine during pregnancy (Chambers et al., 1996 ; Pastuszak et al., 1993
; Pastuszak et al., 1993 ; Nulman et al., 1997
; Nulman et al., 1997 ), increasing the potential for exposure of human
offspring to fluoxetine. Yet, to date, few published studies have
investigated the neurochemical teratogenic potential of this widely
prescribed medication.
), increasing the potential for exposure of human
offspring to fluoxetine. Yet, to date, few published studies have
investigated the neurochemical teratogenic potential of this widely
prescribed medication.
In humans, the research to date suggests that use of fluoxetine during
embryogenesis neither increases the risk of fetal malformations nor
produces behavioral abnormalities in preschool age children (Nulman
and Koren, 1996 ; Nulman et al., 1997
; Nulman et al., 1997 ; Pastuszak et al., 1993
; Pastuszak et al., 1993 ). However, one study reports contradictory findings following
drug exposure throughout the entire term of pregnancy (Chambers
et al., 1996
). However, one study reports contradictory findings following
drug exposure throughout the entire term of pregnancy (Chambers
et al., 1996 ). In general, the findings in humans indicating a lack
of effect on offspring vital parameters, physical appearance, or
behavioral measures following prenatal exposure to fluoxetine are
supported by data from animal studies (Byrd and Markham, 1994
). In general, the findings in humans indicating a lack
of effect on offspring vital parameters, physical appearance, or
behavioral measures following prenatal exposure to fluoxetine are
supported by data from animal studies (Byrd and Markham, 1994 ; Cabrera and Battaglia, 1994
; Cabrera and Battaglia, 1994 ; Hoyt et al., 1989
; Hoyt et al., 1989 ; Vorhees et al., 1994
; Vorhees et al., 1994 ). However, in contrast to the absence of obvious physical
or behavioral anomalies following prenatal fluoxetine exposure,
other evidence in rats indicates that prenatal exposure to fluoxetine
can produce biochemical alterations in brain 5-HT systems in both
immature and adult offspring (Cabrera et al., 1994
). However, in contrast to the absence of obvious physical
or behavioral anomalies following prenatal fluoxetine exposure,
other evidence in rats indicates that prenatal exposure to fluoxetine
can produce biochemical alterations in brain 5-HT systems in both
immature and adult offspring (Cabrera et al., 1994 ; Cabrera-Vera et al., 1997
; Cabrera-Vera et al., 1997 ; Montero et al., 1990
; Montero et al., 1990 ; Romero et al., 1994
; Romero et al., 1994 ). These studies are consistent with data demonstrating
that both fluoxetine and its active metabolite, norfluoxetine, cross
the placenta and enter fetal brain tissue (Pohland et al.,
1989
). These studies are consistent with data demonstrating
that both fluoxetine and its active metabolite, norfluoxetine, cross
the placenta and enter fetal brain tissue (Pohland et al.,
1989 ). After infiltrating fetal brain tissue, fluoxetine may
alter extracellular concentrations of 5-HT by interacting with 5-HT
transporters present on developing neurons and glia. Recent studies
indicate that 5-HT exerts a trophic influence on the outgrowth and
targeting of 5-HT neuronal projections and on the maturation of 5-HT
target tissues (Lauder, 1990
). After infiltrating fetal brain tissue, fluoxetine may
alter extracellular concentrations of 5-HT by interacting with 5-HT
transporters present on developing neurons and glia. Recent studies
indicate that 5-HT exerts a trophic influence on the outgrowth and
targeting of 5-HT neuronal projections and on the maturation of 5-HT
target tissues (Lauder, 1990 ; Whitaker-Azmitia et al., 1996
; Whitaker-Azmitia et al., 1996 ). Therefore, our hypothesis was that the disruption of
5-HT systems during fetal brain development by the administration of
fluoxetine would result in biochemical alterations in brain 5-HT
pathways in offspring.
). Therefore, our hypothesis was that the disruption of
5-HT systems during fetal brain development by the administration of
fluoxetine would result in biochemical alterations in brain 5-HT
pathways in offspring.
We previously reported that prenatal exposure to fluoxetine, at the same dose
used in this study, produces site-specific alterations in brain 5-HT
content (Cabrera-Vera et al., 1997 ), 5-HT2A/2C receptor density, and
5-HT2A/2C receptor-mediated hormone secretion in the
absence of visually apparent physical abnormalities (Cabrera and
Battaglia, 1994
), 5-HT2A/2C receptor density, and
5-HT2A/2C receptor-mediated hormone secretion in the
absence of visually apparent physical abnormalities (Cabrera and
Battaglia, 1994 ). The purpose of the present study was to determine
whether prenatal exposure to fluoxetine would alter serotonergic
innervation of various brain regions, as assessed from densities of
5-HT transporters (Battaglia et al., 1987
). The purpose of the present study was to determine
whether prenatal exposure to fluoxetine would alter serotonergic
innervation of various brain regions, as assessed from densities of
5-HT transporters (Battaglia et al., 1987 ; Descarries et al., 1995
; Descarries et al., 1995 ). In vitro autoradiography was employed to determine
changes in 5-HT transporters in specific neuroanatomic regions,
as our previous data indicated that alterations in brain 5-HT
systems may be subtle and localized to discrete neuroanatomic
loci (Cabrera-Vera et al., 1997
). In vitro autoradiography was employed to determine
changes in 5-HT transporters in specific neuroanatomic regions,
as our previous data indicated that alterations in brain 5-HT
systems may be subtle and localized to discrete neuroanatomic
loci (Cabrera-Vera et al., 1997 ). In addition, since our previous research indicated
that prenatal exposure to fluoxetine can alter brain 5-HT pathways in
an age-specific manner (Cabrera-Vera et al., 1997
). In addition, since our previous research indicated
that prenatal exposure to fluoxetine can alter brain 5-HT pathways in
an age-specific manner (Cabrera-Vera et al., 1997 ; Cabrera and Battaglia, 1994
; Cabrera and Battaglia, 1994 ), the present study determined the density of 5-HT
transporters at both prepubescent and adult ages. The data reported
herein provide additional biochemical evidence that prenatal exposure
to fluoxetine can alter select serotonergic pathways in offspring,
and that these changes are age-dependent and discretely localized to
specific neuroanatomic loci.
), the present study determined the density of 5-HT
transporters at both prepubescent and adult ages. The data reported
herein provide additional biochemical evidence that prenatal exposure
to fluoxetine can alter select serotonergic pathways in offspring,
and that these changes are age-dependent and discretely localized to
specific neuroanatomic loci.
| |
Methods |
|---|
Animals. Pregnant Sprague-Dawley rats weighing 280-320 g were obtained from Zivic Miller (Zelienople, PA) and maintained in a temperature (22-24°C), humidity (50-55%)- and illumination (12:12 hr light/dark cycle, lights on at 7 a.m.)-controlled facility. The determination of gestational day zero was carried out by the supplier, and was defined by the presence of a copulatory plug. All procedures were conducted in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institute of Health.
Dams. Gravid rats arrived in the laboratory on gestational day
5. Starting on gestational day 8 (GD 8), and continuing
throughout the injection period, all experimental animals were placed
on a nutritionally balanced liquid diet (Riley et al., 1979 ). Experimental dams received injections of either 0.9%
saline (2 ml/kg, s.c.), or fluoxetine hydrochloride
(10 mg/kg/2 ml, s.c.) once daily (at 9 a.m.) beginning on
GD 13, and ending on GD 20. After termination of the
injection paradigm, all animals had free access to food and water.
This exposure period (E13-20) represents a gestational time during
which 5-HT neurons are rapidly differentiating, proliferating and
sending out axonal projections to their respective target regions
(Aitken and Tork, 1988
). Experimental dams received injections of either 0.9%
saline (2 ml/kg, s.c.), or fluoxetine hydrochloride
(10 mg/kg/2 ml, s.c.) once daily (at 9 a.m.) beginning on
GD 13, and ending on GD 20. After termination of the
injection paradigm, all animals had free access to food and water.
This exposure period (E13-20) represents a gestational time during
which 5-HT neurons are rapidly differentiating, proliferating and
sending out axonal projections to their respective target regions
(Aitken and Tork, 1988 ; Lidov and Molliver, 1982
; Lidov and Molliver, 1982 ). Therefore, the rapid development of the 5-HT system
from GD 13 through birth makes this period of development
particularly sensitive to perturbations in the serotonergic system.
). Therefore, the rapid development of the 5-HT system
from GD 13 through birth makes this period of development
particularly sensitive to perturbations in the serotonergic system.
 ) that this treatment paradigm does not alter either maternal
weight gain or nutritional intake of the pregnant dams. In
addition, 10 mg/kg fluoxetine administered once daily represents
a treatment paradigm commonly utilized to assess the effects of
repeated fluoxetine administration on adult animals (Li et
al., 1993
) that this treatment paradigm does not alter either maternal
weight gain or nutritional intake of the pregnant dams. In
addition, 10 mg/kg fluoxetine administered once daily represents
a treatment paradigm commonly utilized to assess the effects of
repeated fluoxetine administration on adult animals (Li et
al., 1993 ).
).
Offspring. At birth (i.e., postnatal day 0, PD 0), all
offspring from each of the experimental groups were fostered to untreated,
lactating dams in order to eliminate the possible influence of
drug-induced differences in nurturing. At the time of fostering, pups
(from dams of 10 or more pups per litter), were culled to
9 pups per litter (5 males, 4 females) until weaning.
As we previously reported in detail using an identical treatment
paradigm (Cabrera and Battaglia, 1994 ), fluoxetine exposed offspring do not exhibit any visually
apparent physical abnormalities. Furthermore, there were no
significant differences in fetal viability between control and
fluoxetine-exposed litters (Cabrera and Battaglia, 1994
), fluoxetine exposed offspring do not exhibit any visually
apparent physical abnormalities. Furthermore, there were no
significant differences in fetal viability between control and
fluoxetine-exposed litters (Cabrera and Battaglia, 1994 ). All pups were weaned on PD 21. The number of
samples (N) within each group was comprised of single pups obtained
from different litters. At the time of weaning, males were housed in
groups of 2 or 3 rats per cage, and had free access to food
and water. The density of serotonin uptake sites was determined in
male progeny at either PD 28 or PD 70; time points representing
pre- and postpubescent ages, respectively. The rationale to focus the
present studies to male offspring was 2-fold: (1) for comparison with
other studies investigating the effects of comparable doses of
fluoxetine administered to adult male rats, and (2) to preclude the
potential confounding influence of differing ovarian hormone levels
on transporter densities in adult female offspring which may not be
cycling in syncrony. In addition, these postnatal ages were chosen
based on our previous work indicating that prenatal exposure to
fluoxetine produces differential changes in pre- and postsynaptic
components of 5-HT systems at adult (PD 70) vs. prepubescent
(PD 28) ages (Cabrera and Battaglia, 1994
). All pups were weaned on PD 21. The number of
samples (N) within each group was comprised of single pups obtained
from different litters. At the time of weaning, males were housed in
groups of 2 or 3 rats per cage, and had free access to food
and water. The density of serotonin uptake sites was determined in
male progeny at either PD 28 or PD 70; time points representing
pre- and postpubescent ages, respectively. The rationale to focus the
present studies to male offspring was 2-fold: (1) for comparison with
other studies investigating the effects of comparable doses of
fluoxetine administered to adult male rats, and (2) to preclude the
potential confounding influence of differing ovarian hormone levels
on transporter densities in adult female offspring which may not be
cycling in syncrony. In addition, these postnatal ages were chosen
based on our previous work indicating that prenatal exposure to
fluoxetine produces differential changes in pre- and postsynaptic
components of 5-HT systems at adult (PD 70) vs. prepubescent
(PD 28) ages (Cabrera and Battaglia, 1994 ; Cabrera-Vera et al., 1997
; Cabrera-Vera et al., 1997 ). Therefore, as the effects of fluoxetine exposure on
brain 5-HT systems have repeatedly demonstrated age-dependent
specificity, the current study examined the same two developmental
ages to determine whether prenatal fluoxetine exposure also produces
age-dependent changes in brain 5-HT transporters.
). Therefore, as the effects of fluoxetine exposure on
brain 5-HT systems have repeatedly demonstrated age-dependent
specificity, the current study examined the same two developmental
ages to determine whether prenatal fluoxetine exposure also produces
age-dependent changes in brain 5-HT transporters.
Autoradiographic localization of 5-HT transporters. Rats were
sacrificed by decapitation, their brains were quickly removed, frozen on
powdered dry ice, secured with parafilm and plastic wrap then stored
at 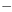 70°C. Coronal sections of brains were obtained at
70°C. Coronal sections of brains were obtained at 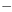 20°C using a cryostat (Hacker Instruments, Inc.) set
to obtain sections 15 µm thick. Sections were thaw-mounted onto
chrome alum/gelatin-coated microscope slides, and stored at
20°C using a cryostat (Hacker Instruments, Inc.) set
to obtain sections 15 µm thick. Sections were thaw-mounted onto
chrome alum/gelatin-coated microscope slides, and stored at 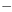 20°C until used for autoradiographic measurement of 5-HT
transporters. Coronal sections were taken at the following
8 levels according to the rat atlas by Paxinos and Watson
(1986)
20°C until used for autoradiographic measurement of 5-HT
transporters. Coronal sections were taken at the following
8 levels according to the rat atlas by Paxinos and Watson
(1986) : Bregma +3.70 mm, +1.00 mm,
: Bregma +3.70 mm, +1.00 mm, 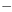 0.30 mm,
0.30 mm, 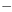 1.80 mm,
1.80 mm, 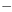 2.80 mm,
2.80 mm, 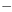 3.14 mm,
3.14 mm, 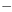 4.80 mm and
4.80 mm and 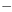 8.00 mm.
8.00 mm.
 . Slide mounted sections were preincubated at room
temperature for 15 min in 50 mM Tris HCl containing 120 mM
NaCl and 5 mM KCl (pH 7.7 at 25°C). Sections were then
incubated at room temperature in 0.7 nM
[3H]citalopram (specific activity 81 Ci/mmol;
KD = 0.84 nM) in the absence or
presence of 1 µM paroxetine to determine nonspecific binding.
Following incubation, sections were washed twice in ice cold
50 mM Tris HCl containing 120 mM NaCl and 5 mM KCl (pH 7.7
at 25°C) for 10 min each time then dipped in ice cold deionized
water, and dried rapidly under a stream of cold dry air. Following
the in vitro labeling of 5-HT transporters, the slides were
dried overnight in slide boxes containing desiccant prior to
apposing them to tritium sensitive film (Hyperfilm-3H;
Amersham Corporation, Arlington Heights, IL) at 4°C for 30 days to
generate the autoradiograms. Slides containing low and high tritium
standards (Amersham Corporation) were included with each film to
control for exposure differences between films. The films were
developed by placing them in Kodak Developer D-19 for 5 min,
then in Kodak Indicator Stop Bath solution for 30 sec, followed
by 5 min in Kodak Fixer. The films were subsequently placed in a
running water bath containing Kodak Photo-Flo 200 Solution for
15 min, then air dried. Autoradiograms were quantitated on a
Macintosh Quadra 950 computer using the public domain NIH Image
program version 1.55 (developed at the U.S. National Institutes
of Health and available from the Internet by anonymous FTP from
zippy.nimh.nih.gov). Standard
curves of film grey scale values and radioactivity generated by
tritium standards coexposed with labeled slides, was best fit by an
exponential function. Grey scale values were converted to units of
radioactivity and then to fmol of sites/mg tissue equivalent taking
into account the specific activity of the radioligand.
. Slide mounted sections were preincubated at room
temperature for 15 min in 50 mM Tris HCl containing 120 mM
NaCl and 5 mM KCl (pH 7.7 at 25°C). Sections were then
incubated at room temperature in 0.7 nM
[3H]citalopram (specific activity 81 Ci/mmol;
KD = 0.84 nM) in the absence or
presence of 1 µM paroxetine to determine nonspecific binding.
Following incubation, sections were washed twice in ice cold
50 mM Tris HCl containing 120 mM NaCl and 5 mM KCl (pH 7.7
at 25°C) for 10 min each time then dipped in ice cold deionized
water, and dried rapidly under a stream of cold dry air. Following
the in vitro labeling of 5-HT transporters, the slides were
dried overnight in slide boxes containing desiccant prior to
apposing them to tritium sensitive film (Hyperfilm-3H;
Amersham Corporation, Arlington Heights, IL) at 4°C for 30 days to
generate the autoradiograms. Slides containing low and high tritium
standards (Amersham Corporation) were included with each film to
control for exposure differences between films. The films were
developed by placing them in Kodak Developer D-19 for 5 min,
then in Kodak Indicator Stop Bath solution for 30 sec, followed
by 5 min in Kodak Fixer. The films were subsequently placed in a
running water bath containing Kodak Photo-Flo 200 Solution for
15 min, then air dried. Autoradiograms were quantitated on a
Macintosh Quadra 950 computer using the public domain NIH Image
program version 1.55 (developed at the U.S. National Institutes
of Health and available from the Internet by anonymous FTP from
zippy.nimh.nih.gov). Standard
curves of film grey scale values and radioactivity generated by
tritium standards coexposed with labeled slides, was best fit by an
exponential function. Grey scale values were converted to units of
radioactivity and then to fmol of sites/mg tissue equivalent taking
into account the specific activity of the radioligand.
Drugs. [3H]Citalopram (81 Ci/mmol) was obtained from New England Nuclear (Boston, MA). Paroxetine was a generous gift from SmithKline Beecham Pharmaceuticals (Philadelphia, PA). Fluoxetine was a generous gift from Eli Lilly and Co. (Indianapolis, IN). All other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Statistics. The data are represented as the group means and the S.E.M. Statistical analysis of the data was performed by two-way analysis of variance (ANOVA). If the F values from the ANOVA indicated significant differences, individual group means were then compared by Newman-Keuls test using a computer program (SigmaStat, San Rafael, CA).
| |
Results |
|---|
Changes in the Density of 5-HT transporters Following Prenatal Fluoxetine Exposure
Telencephalon. Site-specific alterations in 5-HT transporters were observed in specific telencephalic brain regions following prenatal exposure to fluoxetine (table 1, fig. 1). For example, 5-HT transporters were significanty (P < .05; Neuman-Keuls test) increased only in the CA2 (+47%) and CA3 (+38%) areas of the hippocampus in fluoxetine-exposed prepubescent offspring, whereas the density of 5-HT transporters was not altered in either the dentate gyrus or in the CA1 area of the hippocampus (table 1). 5-HT transporters were also significantly elevated in select nuclei of the amygdala in prepubescent offspring prenatally exposed to fluoxetine. For example, 5-HT transporters were significantly increased in the basolateral (+32%; F(1,13) = 6.05, P = .029) and medial (+44%; F(1,15) = 6.45, P = .023) amygdaloid nuclei, as determined by two-way ANOVA. However, 5-HT transporters in the central amygdala, caudate putamen, globus pallidus and ventral pallidum were similar between control and fluoxetine-exposed prepubescent offspring. In contrast, in adult offspring (table 1), prenatal exposure to fluoxetine did not significantly alter the density of 5-HT transporters in any subregion of the hippocampus (CA1, CA2, CA3, dentate) nor in any of the basal ganglia or amygdaloid nuclei examined in the present study (central, basolateral, and medial amygdala; caudate putamen, globus pallidus, and ventral pallidum). In either prepubescent or adult progeny, prenatal exposure to fluoxetine did not alter the density of 5-HT transporters in any of the cortical areas examined (cingulate, frontal, entorhinal, occipital, parietal, retrosplenial granular and temporal cortex). Similarly, the density of 5-HT transporters in lateral septal nuclei (dorsal and intermediate areas) was not affected by prenatal fluoxetine exposure in either prepubescent or adult progeny.
|
|
|
Diencephalon and mesencephalon. As observed in the telencephalon,
changes in 5-HT transporters were observed in select regions of diencephalon and
mesencephalon only in prepubescent progeny (table 2, fig.
1).
However, both increases and decreases in 5-HT transporters were
detected. For example, two-way ANOVA indicated a significant
elevation in 5-HT transporters in the lateral hypothalamus (+21%;
F(1,15) = 10.14, P = .006) in
prepubescent fluoxetine-exposed progeny. In contrast, a significant
reduction (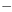 21%; Neuman-Keuls test, P < .05) in 5-HT
transporter density was detected in the dorsomedial hypothalamic
nucleus of prepubescent progeny. However, densities of 5-HT
transporters were similar between control and fluoxetine-exposed
animals in all other subregions of the hypothalamus examined
(anterior, arcuate, paraventricular and ventromedial nuclei; medial
mammillary and medial preoptic areas) in prepubescent progeny. In
addition to hypothalamic alterations, two-way ANOVA indicated that
prenatal exposure to fluoxetine produced a significant decrease in
5-HT transporters in the substantia nigra (
21%; Neuman-Keuls test, P < .05) in 5-HT
transporter density was detected in the dorsomedial hypothalamic
nucleus of prepubescent progeny. However, densities of 5-HT
transporters were similar between control and fluoxetine-exposed
animals in all other subregions of the hypothalamus examined
(anterior, arcuate, paraventricular and ventromedial nuclei; medial
mammillary and medial preoptic areas) in prepubescent progeny. In
addition to hypothalamic alterations, two-way ANOVA indicated that
prenatal exposure to fluoxetine produced a significant decrease in
5-HT transporters in the substantia nigra (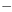 19%; F(1,12) = 9.76, P
= .0007) in prepubescent offspring. Despite the alterations in
5-HT transporters in a number of regions receiving serotonergic
innervation, 5-HT transporters, in brain regions composed primarily
of serotonin perikarya (i.e., dorsal and median raphe nuclei),
were not altered by prenatal exposure to fluoxetine.
19%; F(1,12) = 9.76, P
= .0007) in prepubescent offspring. Despite the alterations in
5-HT transporters in a number of regions receiving serotonergic
innervation, 5-HT transporters, in brain regions composed primarily
of serotonin perikarya (i.e., dorsal and median raphe nuclei),
were not altered by prenatal exposure to fluoxetine.
|
Changes in the density of 5-HT transporters as a consequence of
maturation. The regional specificity of changes in the density of 5-HT
transporters as a consequence of normal maturation (i.e.,
prepubescent vs. adult densities of 5-HT transporters in
control progeny) are shown in figure 2.
While, the majority of brain areas examined in the present study
exhibited comparable densities of 5-HT transporters at prepubescent
and adult ages (tables 1 and 2),
notable increases and decreases were observed in several specific
neuroanatomic loci. For example, adult control progeny exhibited a
significantly greater density of 5-HT transporters than their
prepubescent counterparts in the cingulate cortex (+33%;
F(1,14) = 14.56, P < .0019), the
arcuate nucleus of the hypothalamus (+ 56%;
P < .05, Neuman-Keuls test), the basolateral (+58%;
F(1,13) = 37.81, P < .0001) and
medial (+66%;
F(1,15) = 26.84, P = .0001) amygdaloid
nuclei, as well as in CA1 (+48%;
F(1,16) = 14.68, P = .0015) and CA3
(+76%;
F(1,15) = 27.86, P < .0001) areas
of the hippocampus (tables 1
and 2). In
contrast, significant age-related reductions were noted in a number
of other brain regions (tables 1 and 2;
fig. 2).
Two-way ANOVA indicated significantly lower densities of 5-HT
transporters in control adult offspring within the temporal cortex
(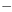 65%;
F(1,16) = 18.91, P = .0005), substantia
nigra (
65%;
F(1,16) = 18.91, P = .0005), substantia
nigra (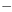 26%; F(1,12)
= 20.12, P = .0007), ventral tegmental area (
26%; F(1,12)
= 20.12, P = .0007), ventral tegmental area (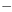 31%; F(1,16) = 19.08,
P = 0.0005), and median raphe (
31%; F(1,16) = 19.08,
P = 0.0005), and median raphe (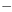 25%;
F(1,14) = 14.56, P = .0019).
These changes may represent changes in 5-HT innervation or functional
changes in 5-HT neurons in these regions as a consequence of normal
maturation.
25%;
F(1,14) = 14.56, P = .0019).
These changes may represent changes in 5-HT innervation or functional
changes in 5-HT neurons in these regions as a consequence of normal
maturation.
| |
Discussion |
|---|
The present study demonstrates that prenatal exposure to fluoxetine produces region-specific alterations in the density of [3H]citalopram-labeled 5-HT transporters in prepubescent offspring. In particular, 5-HT transporter densities were markedly altered in the substantia nigra, as well as in several brain regions which are integral components of the limbic system including subregions of the hippocampus, amygdala, and hypothalamus. The increases and decreases in [3H]citalopram-labeled 5-HT transporters in offspring reflects fluoxetine-induced alterations in either: (1) the extent of serotonergic innervation; (2) the number of transporters present per neuron; or (3) the affinity of the radiolabel for the transporter.
Through the years, many studies have suggested a link between 5-HT
innervation density and the amount of specific radiolabeled 5-HT
transporters in brain tissue (Battaglia et al., 1987 ; Battaglia, 1990
; Battaglia, 1990 ; D'Amato et al., 1987
; D'Amato et al., 1987 ; Pranzatelli and Martens, 1992
; Pranzatelli and Martens, 1992 ). More recently, Descarries et al. (1995)
). More recently, Descarries et al. (1995) reported that quantitative autoradiography of
[3H]citalopram-labeled 5-HT transporters in rat brain slices,
paralleled treatment induced changes in the density of 5-HT
innervation as measured by the number of [3H]5-HT-labeled
varicosities. Consistent with previous research, these authors
concluded that radiolabeled citalopram could serve as a quantitative
marker for 5-HT innervation in vitro. Thus, it is likely that
alterations in [3H]citalopram-labeled 5-HT transporters in
prepubescent offspring reflect drug-induced changes in the extent of
serotonergic innervation of the affected brain regions. However, data
in transfected cell lines also suggests that the number of 5-HT
transporters present at the cell surface can be regulated by the
stimulation of protein kinases in a similar way to what has more
traditionally been described for the 5-HT receptors (Qian et
al., 1997
reported that quantitative autoradiography of
[3H]citalopram-labeled 5-HT transporters in rat brain slices,
paralleled treatment induced changes in the density of 5-HT
innervation as measured by the number of [3H]5-HT-labeled
varicosities. Consistent with previous research, these authors
concluded that radiolabeled citalopram could serve as a quantitative
marker for 5-HT innervation in vitro. Thus, it is likely that
alterations in [3H]citalopram-labeled 5-HT transporters in
prepubescent offspring reflect drug-induced changes in the extent of
serotonergic innervation of the affected brain regions. However, data
in transfected cell lines also suggests that the number of 5-HT
transporters present at the cell surface can be regulated by the
stimulation of protein kinases in a similar way to what has more
traditionally been described for the 5-HT receptors (Qian et
al., 1997 ). Hence, it is also possible that the reductions in
[3H]citalopram-labeled transporters produced by prenatal
fluoxetine may reflect changes in the number of transporters present
per neuron within a brain region, rather than alterations in the
extent of serotonergic innervation. Alterations in the number of
5-HT transporter sites per neuron could result from changes in
the stability of the protein or intracellular trafficking and
insertion into the plasma membrane. Finally, the present study
utilized a single concentration of radioligand below the
KD value for the radiolabel to assess 5-HT
transporter binding. Because this approach renders radioligand
binding sensitive to changes in either the affinity and/or density of
5-HT transporters, we cannot rule out the possibility that prenatal
fluoxetine exposure altered the affinity of the transport protein for
the radioligand in 28-day-old offspring. However, this possibility is
unlikely, as one would expect that changes in the affinity of the
transporter would result in unidirectional changes (either all
increases or all decreases) in citalopram-labeling of 5-HT
transporters which would persist into adulthood. However, we report
both increases and decreases in 5-HT transporters in specific nuclei
which are present at prepubescent but not adult ages. Consistent with
this contention, Montero et al. (1990)
). Hence, it is also possible that the reductions in
[3H]citalopram-labeled transporters produced by prenatal
fluoxetine may reflect changes in the number of transporters present
per neuron within a brain region, rather than alterations in the
extent of serotonergic innervation. Alterations in the number of
5-HT transporter sites per neuron could result from changes in
the stability of the protein or intracellular trafficking and
insertion into the plasma membrane. Finally, the present study
utilized a single concentration of radioligand below the
KD value for the radiolabel to assess 5-HT
transporter binding. Because this approach renders radioligand
binding sensitive to changes in either the affinity and/or density of
5-HT transporters, we cannot rule out the possibility that prenatal
fluoxetine exposure altered the affinity of the transport protein for
the radioligand in 28-day-old offspring. However, this possibility is
unlikely, as one would expect that changes in the affinity of the
transporter would result in unidirectional changes (either all
increases or all decreases) in citalopram-labeling of 5-HT
transporters which would persist into adulthood. However, we report
both increases and decreases in 5-HT transporters in specific nuclei
which are present at prepubescent but not adult ages. Consistent with
this contention, Montero et al. (1990) identified reductions in [3H]imipramine-labeled 5-HT transporters
following prenatal fluoxetine exposure in the absence of alterations
in the affinity of the ligand for the transport protein.
identified reductions in [3H]imipramine-labeled 5-HT transporters
following prenatal fluoxetine exposure in the absence of alterations
in the affinity of the ligand for the transport protein.
As previously discussed, the underlying mechanism for the reductions in the
density of [3H]citalopram-labeled 5-HT transporters cannot be
definitively concluded from the present study. However, regardless of
the mechanism responsible for the differences in 5-HT transporter
density (i.e. alterations in the extent of serotonergic
innervation, or in the number of 5-HT transporters per nerve
terminal), one can speculate that fluoxetine-induced changes in 5-HT
transporter numbers may result in alterations in serotonergic
neurotransmission within the brain regions affected by the prenatal
treatment. This possibility is supported by the fact that the 5-HT
transporter plays a key role in regulating extracellular
concentrations of 5-HT and consequent receptor activation (Schroeter
and Blakely, 1996 ).
).
The prenatal fluoxetine-induced alterations in
[3H]citalopram-labeled 5-HT transporters appears to be age-dependent,
since no significant differences in the density of 5-HT
transporters were observed in adult offspring. In this regard, the
present studies are consistent with the age-dependent changes in the
density of [3H]imipramine-labeled 5-HT transporters
reported by Montero et al. (1990) following prenatal exposure to fluoxetine (2.5 mg/kg/day).
However, the present data appear to contrast with our previous
report indicating that prenatal fluoxetine exposure did not alter
the density of 5-HT transporters in homogenates of various forebrain
regions (Cabrera and Battaglia, 1994
following prenatal exposure to fluoxetine (2.5 mg/kg/day).
However, the present data appear to contrast with our previous
report indicating that prenatal fluoxetine exposure did not alter
the density of 5-HT transporters in homogenates of various forebrain
regions (Cabrera and Battaglia, 1994 ; Cabrera-Vera et al., 1997
; Cabrera-Vera et al., 1997 ). Taken together, these studies indicate that prenatal
fluoxetine exposure produces subtle region-specific alterations in
select brain 5-HT pathways that can not be readily discerned from
homogenate assays, which are more likely to detect global, rather
than discrete, changes in neurotransmitter systems within specific
brain regions.
). Taken together, these studies indicate that prenatal
fluoxetine exposure produces subtle region-specific alterations in
select brain 5-HT pathways that can not be readily discerned from
homogenate assays, which are more likely to detect global, rather
than discrete, changes in neurotransmitter systems within specific
brain regions.
While differences in tritium quenching between prepubescent and adult progeny
(due to age-dependent changes in lipid composition of the brain)
could be postulated to produce artifactual differences between
prepubescent and adult offspring 5-HT transporter densities, this is
unlikely to have contributed to the autoradiographic differences
reported herein. This contention is supported by several
observations: (1) autoradiographic analysis of adjacent sections with
other tritiated radioligands does not reveal the presence of a
consistent pattern of quenching across the two age groups in any
specific brain region (Cabrera et al., 1995 ); (2) the majority of the radioactive signal obtained
in the regions analyzed in the present study results from
localization of the transporter in 5-HT neuronal cell bodies or
terminals rather than from axons of passage; (3) both age-related
increases and decreases in [3H]citalopram-labeled transporters
were observed, whereas developmental delays in myelination would be
expected to alter the signal in the same direction across all brain
regions. In addition, developmental differences in the pattern of
3H-citalopram-labeled 5-HT transporters in prepubescent rats has
previously been reported (D'Amato et al., 1987
); (2) the majority of the radioactive signal obtained
in the regions analyzed in the present study results from
localization of the transporter in 5-HT neuronal cell bodies or
terminals rather than from axons of passage; (3) both age-related
increases and decreases in [3H]citalopram-labeled transporters
were observed, whereas developmental delays in myelination would be
expected to alter the signal in the same direction across all brain
regions. In addition, developmental differences in the pattern of
3H-citalopram-labeled 5-HT transporters in prepubescent rats has
previously been reported (D'Amato et al., 1987 ).
).
The absence of changes in 5-HT transporters in adult animals prenatally
exposed to fluoxetine observed in the present study may initially
suggest that 5-HT systems have "normalized" by adulthood. However,
functional deficits in 5-HT nerve terminals may be present in adult
animals in the absence of alterations in the density of 5-HT
transporters. Consistent with this hypothesis, Battaglia (1990) demonstrated that, in cortex of rats recovering from MDMA-induced
lesion of serotonergic axons, following an initial 90% reduction
in 5-HT transporters, 5-HT transporters reached control levels
after 1 year. However, 5-HT levels remained markedly below
control values suggesting a functional alteration in presynaptic
5-HT neurons. Furthermore, we recently reported that midbrain
5-HT content was significantly reduced in adult progeny
prenatally exposed to fluoxetine (Cabrera-Vera et al., 1997
demonstrated that, in cortex of rats recovering from MDMA-induced
lesion of serotonergic axons, following an initial 90% reduction
in 5-HT transporters, 5-HT transporters reached control levels
after 1 year. However, 5-HT levels remained markedly below
control values suggesting a functional alteration in presynaptic
5-HT neurons. Furthermore, we recently reported that midbrain
5-HT content was significantly reduced in adult progeny
prenatally exposed to fluoxetine (Cabrera-Vera et al., 1997 ). This reduction in 5-HT content occurred in the
absence of concomitant changes in the density of dorsal and median
raphe 5-HT transporters as reported in the present studies. We have
also previously reported a significant attenuation of the ability of
the 5-HT releasing drug PCA to reduce 5-HT content in midbrain of
adult offspring prenatally exposed to fluoxetine; providing further
evidence of a functional impairment in 5-HT neurons in this region
(Cabrera-Vera et al., 1997
). This reduction in 5-HT content occurred in the
absence of concomitant changes in the density of dorsal and median
raphe 5-HT transporters as reported in the present studies. We have
also previously reported a significant attenuation of the ability of
the 5-HT releasing drug PCA to reduce 5-HT content in midbrain of
adult offspring prenatally exposed to fluoxetine; providing further
evidence of a functional impairment in 5-HT neurons in this region
(Cabrera-Vera et al., 1997 ). Taken together, these data suggest that whereas there
may be a recovery from the changes in 5-HT transporter densities in
prepubescent rats following maturation, the functional status of 5-HT
terminals in brain regions affected by prenatal fluoxetine exposure
may remain compromised in adult progeny.
). Taken together, these data suggest that whereas there
may be a recovery from the changes in 5-HT transporter densities in
prepubescent rats following maturation, the functional status of 5-HT
terminals in brain regions affected by prenatal fluoxetine exposure
may remain compromised in adult progeny.
Consistent with the hypothesis of functional changes in 5-HT pathways in
adult progeny, we previously reported that prenatal fluoxetine
exposure reduced hypothalamic 5-HT2A/2C receptors and the
5-HT2A/2C receptor-mediated adrenocorticotropin response
selectively in adult, but not prepubescent progeny (Cabrera and
Battaglia, 1994 ). Hence, our previous data suggested a delayed onset of
perturbations in postsynaptic brain 5-HT pathways following prenatal
fluoxetine exposure. According to the classic theory of receptor
regulation, receptor number and/or function is altered secondary to
changes in a presynaptic stimulus. Thus, fluoxetine-induced changes
in postsynaptic 5-HT receptor systems may be due, in part, to
alterations in the extent of serotonergic innervation or to the
altered functional status of 5-HT neurons which occur early in the
life of the offspring.
). Hence, our previous data suggested a delayed onset of
perturbations in postsynaptic brain 5-HT pathways following prenatal
fluoxetine exposure. According to the classic theory of receptor
regulation, receptor number and/or function is altered secondary to
changes in a presynaptic stimulus. Thus, fluoxetine-induced changes
in postsynaptic 5-HT receptor systems may be due, in part, to
alterations in the extent of serotonergic innervation or to the
altered functional status of 5-HT neurons which occur early in the
life of the offspring.
In summary, the present studies provide additional evidence that prenatal exposure to the selective 5-HT uptake inhibitor fluoxetine (Prozac) results in biochemical alterations in brain 5-HT pathways in offspring. The biochemical alterations reported in the present study (i.e., increases and decreases in the density of 5-HT transporters) are region-specific. As the current data suggest that limbic regions are particularly vulnerable to prenatal fluoxetine exposure, further study of serotonergic function in limbic brain regions might prove to be a particularly fruitful avenue of research. In addition, as activation of the limbic system mediates emotional responses, "fight or flight" reactions, as well as food finding and sexual behaviors, limbic-based behavioral examinations may also be warranted. As 5-HT transporters play a key role in regulating extracellular concentrations of 5-HT and thereby influence the duration and extent of 5-HT receptor activation following neurotransmitter release, the present data suggest the potential for functional alterations in serotonergic neurotransmission within select brain regions in young male offspring exposed in utero to fluoxetine. The nature of the functional consequences of these alterations in 5-HT systems, and the implication for drugs which target 5-HT transporters for their therapeutic efficacy, remain to be elucidated. In addition, it remains to be determined whether the observed changes in brain 5-HT pathways, will be generalizable to other SSRIs, and whether human offspring prenatally exposed to fluoxetine might exhibit similar changes in brain 5-HT pathways. Because alterations in brain 5-HT pathways have been implicated in the etiology of a variety of clinical disorders (e.g., depression, aggression, anxiety), one could speculate that should human offspring exhibit neurochemical alterations similar to those described herein, these individuals may be particularly susceptible to developing psychiatric disorders involving dysfunctional 5-HT pathways. Alternatively, prenatal fluoxetine-induced changes in 5-HT systems in human offspring could alter their responsiveness to therapeutic interventions which modulate brain 5-HT systems. While these possibilities are intriguing they will require a substantial amount of additional research to determine their validity.
| |
Acknowledgments |
|---|
The authors thank Dr. Louis D. Van de Kar, Dr. Qian Li and Mr. Wilfred Pinto for their assistance with the experiments and Mrs. Francisca Garcia for her excellent technical assistance.
| |
Footnotes |
|---|
Accepted for publication May 4, 1998.
Received for publication February 2, 1998.
1 This study was supported in part by Loyola University Potts Foundation, DA 07741, and NSF GER-9253875. T.M.C. was a recipient of a National Science Foundation Minority Graduate Fellowship NSF GER-9253875,
2 Present address: Department of Molecular Pharmacology and Biological Chemistry, Northwestern University, 5-555 Searle, 320 E. Superior, Chicago, IL 60611.
Send reprint requests to: George Battaglia, Ph.D., Department of Pharmacology, Loyola University of Chicago, Stritch School of Medicine, 2160 South First Avenue, Maywood, IL 60153. E-mail: gbattag@luc.edu
| |
Abbreviations |
|---|
ANOVA, analysis of variance; 5-HT, serotonin; PCA, p-chloroamphetamine; PD, postnatal day; SSRI, selective serotonin reuptake inhibitor.
| |
References |
|---|
This article has been cited by other articles:
 |
J. Kim, K. W. Riggs, and D. W. Rurak STEREOSELECTIVE PHARMACOKINETICS OF FLUOXETINE AND NORFLUOXETINE ENANTIOMERS IN PREGNANT SHEEP Drug Metab. Dispos., February 1, 2004; 32(2): 212 - 221. [Abstract] [Full Text] [PDF] |
||||
|
| |||||
.gif) |
T. F. OBERLANDER, R. E. GRUNAU, C. FITZGERALD, A.-L. ELLWOOD, S. MISRI, D. RURAK, and K. W. RIGGS Prolonged Prenatal Psychotropic Medication Exposure Alters Neonatal Acute Pain Response Pediatr. Res., April 1, 2002; 51(4): 443 - 453. [Abstract] [Full Text] [PDF] |
||||
|
| |||||
| ||||||||||||||||||||||||||||||||||||||||||||